
All about spectroscopy
Isaac Newton (1642-1727) taught us that what we call “white light” is actually a mixture of different colors. Spectroscopy is the technique of breaking light down into its constituent colors, just as raindrops do in a rainbow. The result, known as a spectrum, is not only aesthetically pleasing, but also provides a wealth of information about the physical phenomena that produced the light. Different light sources – a flame, an electric bulb, a calibration lamp, sunlight, etc. – produce different spectra, and even different light sources. – often produce very different spectra…
Light typically travels astronomical distances, without losing the information it contains. Analyzing the light from stars, or any other celestial body, is therefore an extraordinary way of understanding the physical phenomena that take place within them. In fact, astronomical spectroscopy is the “basic tool” of astrophysicists.
One might imagine that this is a complex technique reserved for the privileged few who have access to the great instruments of professional observatories. But it’s not! Even a small amateur astronomy instrument can be used for spectroscopy. The entire Shelyak product range is here to convince you.
For an amateur astronomer, access to spectroscopy means moving on from an astronomy of contemplation – a highly respectable motivation! – to direct measurement and intimate understanding of the workings of celestial bodies. It means opening a radically different window on the sky, and believe us, it’s an exciting adventure…
Let’s talk technology
To obtain the spectrum of a star, all you need to do is install a spectroscope behind a telescope. The telescope’s function is to collect the light from a particular star, and the spectroscope to display this light on a sensor (usually a CCD or CMOS camera). If you’re already familiar with astronomical imaging, the spectroscope actually comes between the telescope and the camera.
Spectroscopy has many applications beyond astronomy, notably in chemistry and industry. Its use in astronomy imposes three specific constraints:
- Firstly, the spectroscope must be installed behind the telescope. Weight and size must therefore be adapted to the mount.
- The second is that you need an automatic pointing and tracking system, to ensure that it’s the star you’re looking at: nothing looks more like a star than another star…
- Thirdly, even behind a telescope, the amount of light collected is small. So you need a highly efficient spectroscope so as not to lose a single grain of light…

What do you get with this type of set-up? We observe a particular star, rather than the entire telescope field of view. We place the star on the spectro’s entrance slit to obtain its spectrum.
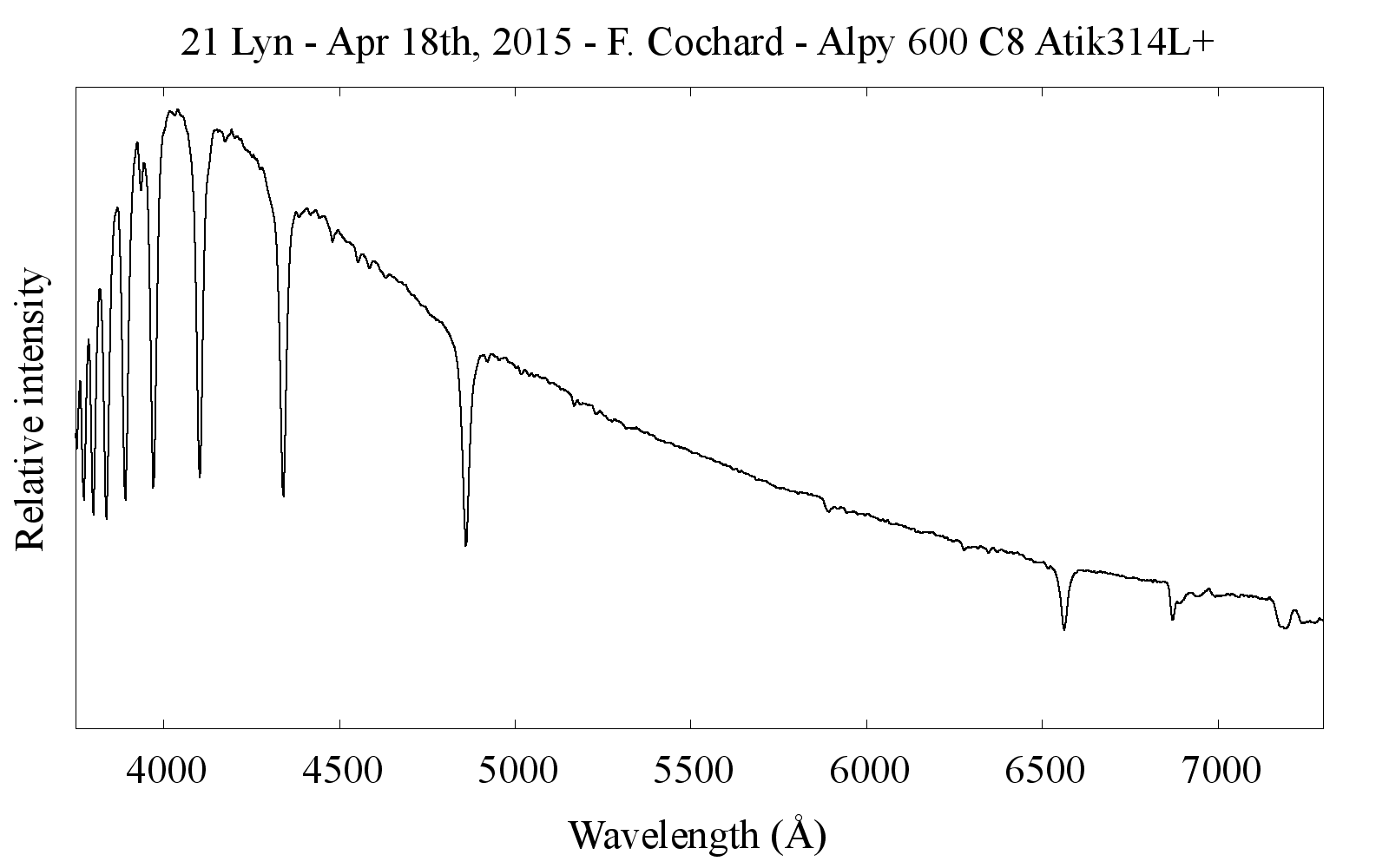
The light spreads out on a line (see left-hand image opposite). Each column of the image corresponds to a wavelength, i.e. a color. Short wavelengths (blue colors) are on the left, long wavelengths (red colors) on the right.
From this raw image, the spectral profile is extracted by a computer process known as “data reduction”. The result is a profile corrected as far as possible for instrumental effects, and calibrated in wavelength (see image on right below).

Relive the history…
Beyond the technical issues, spectroscopy is also a formidable story of human knowledge. It took generations of researchers to understand that the dark (or sometimes light) lines present in a spectrum corresponded to energy transitions in atoms, and that each line was therefore the signature of a chemical element. Or to understand that the laws of physics are the same and apply in the same way throughout the Universe. Light is profoundly linked to matter, so to analyze light is to understand matter. Entering astronomical spectroscopy means embarking on a first-class ticket to a formidable scientific adventure that continues to this day.
… and write the next part!
With access to spectroscopy, you can see for yourself phenomena such as the differences between the spectral types of stars, or the movement of objects by the Doppler effect. You can also use spectroscopy as a teaching tool: the applications are endless. But you can go even further: there’s still a lot to discover, and you yourself can contribute to Research, even with a modest instrument. All you have to do is join the growing community of spectroscopists: the more of us there are, the more useful our observations will be.

